 1.0 INTRODUCTION
1.0 INTRODUCTION
Research on oligomeric proanthocyanidins (OPC) has been ongoing in US, Europe and elsewhere for the past 40 years. The results of this research are very exciting, especially the results of the research on plant extracts including those obtained from pine bark, grape seeds, and whole grape (seed, skin, and stem).
I became involved with the scientific research data on OPC starting in 1989 and designed my first product called PYC-C (standing for pycnogenols and Vitamin C) that same year. It was one of the first products in the US that used pine bark OPCs. At that time, the price for the pine bark extract was $4,000/kg.
In late 1989, I went to a conference on pine bark extract held in France, and I actually visited the pine forest facility where the bark was processed. About a year later, I learned about the availability of grape seed extract which cost less than half the price of pine bark extract and appeared to contain a greater amount of OPC.
During the last 24 years I have designed more than 20 products incorporating OPCs from whole grape and grape seed extracts. Some of these products include: Antioxidant Formula, Blood Sugar Support Formula, Eye & Vision Formula, Joint Health Formula, Hank & Brian’s Mighty Multi-vite!, Mini Multi, Multi Two, Chewable Kid’s Mighty-Multi, OPC-C, PRO-C, Prolyt, Rejuvenate! Pro, Rejuvenate! Berries & Herbs, and Rejuvenate! Strawberry-Peach Protein Plus.
In 1993, I completed a review of the basic science behind OPC that included most of the research of Jack Masquelier the pioneer behind much of the discoveries in this field of research. Around this time scientific articles on OPC began to appear in well known magazines. For example, Morton Walker published articles on this subject in both Raum & Zeit1 and The Townsend Newsletter for Doctors2.
The purpose of this article is to provide details on both the laboratory and clinical research findings concerning these materials. Historically the research literature has used terms such as leucocyanidins, pycnogenols, procyanidins, oligomeric proanthocyanidins, and others to refer to the plant materials which are extracted by a process patented4 by Jack Masquelier about 40 years ago and which has a unique signature when subjected to a test developed by Bate-Smith5.
2.0 BACKGROUND
Before delving deeply into the research findings, I will provide a little background on the structure of bioflavonoids.
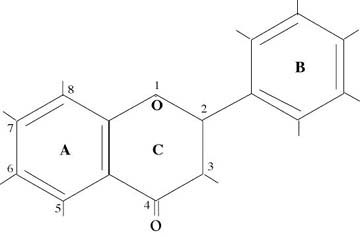
One way of looking at the bioflavonoids is that they are two ring structures (A & B) connected by a three carbon chain (C). Figure 1 shows the general structure of bioflavonoids. The main classes which are distinguished by the types of molecules found at the different numbered positions are FLAVONOL (yellow), ISOFLAVONOL, FLAVONE (yellow), FLAVONONE (colorless), ISOFLAVONONE, ISOFLAVONE (colorless), ANTHOCYANDIN (red, blue, violet), CHALCHONE, and CATECHIN (colorless).
Those classes beginning with the “iso” prefix refer to the structures in which the “B” ring is flipped down from the 2 position to the 3 position. For the anthocyanidins and the catechins the “C” ring is considered to be open which accounts for their greater water solubility. The catechins which are in the class termed flavon-3 ols have an “OH” molecule at the 3, 5, and 7 positions. Depending upon the orientation of the “OH” molecule in the 3 position the material is called catechin (C) or epicatechin (E). In the diagrams these are distinguished by arrows and dashed lines.
Figure 1 — General Structure of Bioflavonoids
The catechins (referring to both catechins and epicatechins) have the peculiar property of forming polymers with themselves. When the number of connected catechins is 10 or less they are called oligomers and thus the term used is “oligomeric proanthocyanidins.” When the number of connected catechins is more than 10 the term condensed tannins is generally used. The term proanthocyanidins comes about because when these materials are subjected to 10% hydrochloric acid and heated to boiling (this is what is termed the Bate-Smith test5), they yield an anthocyanidin, with its intense red coloration, and a catechin.
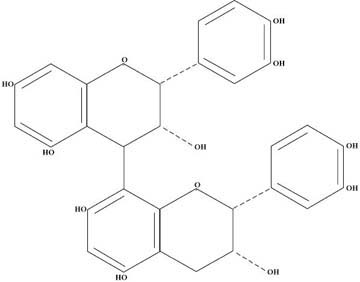
Figure 2 shows a dimeric proanthocyanidin which consists of catechin on top and epicatechin on the bottom. Depending upon which molecule of the lower “A” ring attaches to the “4” position of the upper “C” ring, there are different series of these dimers. The one shown in the diagram fits into what is called the “B” series and is the one of most interest to us here. The dimeric “B” series itself has four members depending whether and where there are catechins and epicatechins, e.g., E-E, C-E, E-C, and C-C. Each of these have been synthesized and studied in the laboratory and have somewhat different chemical properties.
It took almost 20 years for the researchers to come to this understanding concerning the dimers. The situation with respect to trimers, tetramers, pentamers, etc. gets extremely complex and, therefore, much of the research has concentrated upon the dimers or upon materials in which the dimers are the main constituent. This background is sufficient for the reader to understand the bulk of the research results.
Figure 2 — Structure of a Dimeric Proanthocyanidin
3.0 THE EXTRACTION PROCESS
In 1969, Jack Masquelier was granted US Patent No. 3,436,407 regarding the process by which the oligomeric proanthocyanidins could be extracted from raw plant materials in general and pine bark in particular. A flow diagram of the process is shown in Figure 3. The steps of this process insure that the materials obtained are highly water soluble, do not contain condensed tannins, and are not contaminated with toxic substances such as heavy metals, pesticides, and residual solvents.
The proanthocyanidins content of the extracted material is quantitatively assessed by using their property of having a strong affinity for collagen — no other component of the extract having a strong affinity for collagen. In this assessment, a solution of known titre of the extract is prepared and brought in contact with hide powder (collagen). The amount of proanthocyanidins fixed to the hide powder is determined by filtration and weighing, which gives a measure of the original content of the proanthocyanidins in the extract.
The material obtained from pine bark by this process is an astringent tasting light beige powder which can be kept indefinitely in a dry bottle at room temperature. When subjected to high pressure liquid chromatography (HPLC) the constituents shown in Table 1 are typically identified.
|
Table 1. Representative Constituents and Percentages of Pine Bark Extract as Determined by HPLC Analysis
It can be seen from the analysis that the dimeric proanthocyanidins are by far the major component in pine bark extract with a significant amount of the catechin monomer and of other constituents identified to be mostly trimeric and tetrameric proanthocyanidins. Gallic acid, cafeic acid, and ferulic acid are known to have potent in vitro anti-bacterial and anti-viral properties7.
Research investigations8,9,10 have discovered that oligomeric proanthocyanidins (OPCs) may be found in a wide range of other plants materials including:
-
CRAB APPLE
-
APPLE
-
MOUNTAIN CRANBERRY
-
AVOCADO PEAR STONE
-
UNRIPE COCOA BEAN
-
HORSE CHESTNUT (seed shell)
-
HAWTHORNE FRUIT
-
GOAT WILLOW (catkin)
-
UNRIPE STRAWBERRY (fruit, stem)
-
UNRIPE RASPBERRY (fruit, stem)
-
COLA NUTS
-
PEANUT SKINS
-
GRAPE PIPS/SEEDS
-
RED WINE
Commercial production of proanthocyanidins using grape seeds/pips is routinely practiced all over the world. It has been known since the 1960s that red wine is especially high in proanthocyanidins and several companies produce a red wine extract which takes advantage of this fact.
Comparisons of grape pip/seed extract and pine bark extract with regard to their polyphenol content are shown in Table 2.
| PROPERTY | GRAPE PIP/SEED EXTRACT | PINE BARK EXTRACT |
|---|---|---|
| % Polyphenols | 92% | 84% |
| % Monomers (flavan-3-ol) | 32% | 38% |
| % Oligomers (Proanthocyanidins) | 68% | 62% |
| Oligomer/Monomer ratio | 2.1 | 1.6 |
| Polyphenol/Monomer ratio | 3.1 | 2.6 |
| Polyphenol/Oligomer ratio | 1.5 | 1.6 |
Table 2 — Comparison of Polyphenol Content of Grape Pip/Seed and Pine Bark Extracts
Source: “Procyanidins de France” – brochure available from Crossover Marketing 203-481-8863
4.0 BIOCHEMICAL PROPERTIES
4.1 US Patent on Use of Proanthocyanidins
In 1987, Jack Masquelier was granted US Patent No. 4,698,360 titled “Plant Extract with a Proanthocyanidins Content as Therapeutic Agent Having Radical Scavenging Effect and Use Thereof.” The abstract for this patent is given below:
ABSTRACT
“The invention provides a method for preventing and fighting the harmful biological effects of free radicals in the organism of warm blooded animals and more especially human beings, namely cerebral involution, hypoxia following atherosclerosis, cardiac or cerebral infarction, tumor promotion, inflammation, ischaemia, alterations of the synovial fluid, collagen degradation, among others. The method consists of administering ……. an amount, efficient against said effects, of a plant extract with a proanthocyanidins content which has a radical scavenger effect, the extract being in the form of a medicament and coming more especially from the bark of conifers.”
Clearly, Masquelier is claiming a significant therapeutic effect for the proanthocyanidins by means of their potent free-radical scavenging ability. According to the abstract the proanthocyanidins can play a major role in the prevention and cure of a wide range of illnesses. The patent also specifies the dosages which need to be used in order to obtain the therapeutic effects claimed These are:
-
Orally – 1.5 to 3.0 mg daily per kilogram of body weight
-
Intravenously – 5 to 10 mg daily
-
Topically – application of 0.5% ointment one or more times daily
In the following sections the research data which support Masquelier’s claims will be reviewed.
4.2 Bioavailability
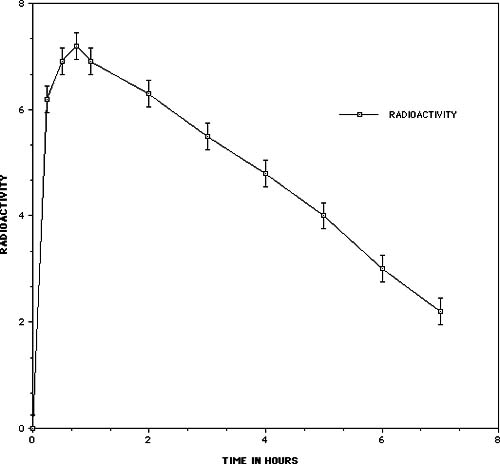
One of the key issues relating to the use of any nutritional supplement or medication is the ability of the substance to get into the body and be utilized at the cellular level. In order to demonstate the bioavailability of the oligomeric proanthocyanidins (PCOs), Masquelier and his colleagues used an isotopic labelling technique11. Grape vines were cultivated in an atmosphere containing 14CO2 for 40 days, during which time each carbon of the flavan molecule became labelled. After extraction and purification the product had an activity of 0.5 µCi per mg. When the PCOs (OPC) were administered orally to a rat or mouse they became rapidly absorbed into the intestinal mucous membrane. The results shown in Figure 3 indicate that radioactivity in the blood was highest after 45 minutes with a half-life in the plasma of 5 hours. Passage into the bile occurs relatively early; within 11 hours almost 14% of the radioactivity was eliminated in this way.
Figure 3 – Radioactivity in the blood of the mouse as a function of time after the administration of 14C labelled oligomeric proanthocyanidins
Radioautographic techniques and measurement of the radioactivity in different organs were used to study the fixation and localization in tissues. The radioautographic cross-sections of the whole mouse showed the distribution of the radioactivity throughout the animal, with a preferential localization in tissues rich in glycosaminoglycans. Measurement of the radioactivity found in various organs is shown in Table 3 and confirms the results of the radioautographic measurements. Radioactivity observed in these experiments was associated with the PCOs and not their degradation products since the fixation takes place in a matter of minutes well before any 14C appears in the exhaled breath.
| ORGAN | RADIOACTIVITY COMPARED WITH TOTAL BLOOD |
|---|---|
| Total Blood | 1.00 |
| Muscle | 1.09 |
| Heart Muscle | 1.18 |
| Plasma | 1.37 |
| Lungs | 1.65 |
| Adrenal Glands | 1.80 |
| Spleen | 2.03 |
| Xyphoid Cartilage | 2.77 |
| Skin | 2.82 |
| Kidney, cortex | 3.57 |
| Trachea | 3.87 |
| Liver | 5.40 |
| Duodenum | 6.75 |
| Aorta | 7.80 |
Table 3 — Radioactivity of Various Organ Tissue Compared With That of the Total Blood
The results of this study showed that the PCOs (OPC) are rapidly absorbed into virtually all of the tissue of mammals (including the brain), and that there is a concentration in tissues high in glycosaminoglycans, namely connective tissues in the skin and organ systems, basement membranes of blood vessels, and cartilage.
4.3 Oligomeric Proanthocyandins Effects on Collagen Fibers
Collagen is the most abundant protein found in the human body. It is the key ingredient in the “glue” that holds us together and consists of helical structures of polypeptides connected together into long chains. Each collagen molecule actually consists of 3 chains, each of which is coiled in a left-handed helix. The three chains are twisted around one another, as the strands of a rope, to form a superhelix. It has been found that Vitamin C is needed in every step of the body processes by which collagen is made. The stability of collagen depends mainly upon the crosslinks which exist between the polypeptide chains.
When collagen fibers are placed into hot water they undergo denaturation and contract rapidly. As the number of crosslinks increase, the contraction temperature increases. An important property of certain polyphenols is that they are able to attach onto collagen and create crosslinks. It is possible to measure the stabilizing effect of these polyphenols by observing the thermal contraction of the fibers onto which they become attached.
In his laboratory, Jack Masquelier conducted a comparison study of the flavonoids with the proanthcyanidins (OPC)11. In the study, reconstituted beef tendon collagen fibers were incubated for 24 hours in an aquous solution of various test substances at a concentration of 1 mg/ml. Each fiber was 10 cm long and supported a weight of 5 grams. When plunged into water at 75°C the reference (untreated) fibers quickly shrank to 4 cm. Table 4 shows shows the contraction time of the various treated fibers, the calculated force of contraction, and the measured amount of the test substance that was actually attached to the collagen fibers.
| TEST SUBSTANCE | CONTRACTION TIME (seconds) | FORCE OF CONTRACTION 10-5W | AMOUNT FIXED PER 100 mg OF COLLAGEN (mg) |
|---|---|---|---|
| Reference Fibers | 10 | 29.4 | 0 |
| Bioflavonoids | 10 | 29.4 | 0 |
| Catechin | 45 | 6.5 | < 10 |
| Condensed Tannin | 70 | 4.2 | 60 |
| Oligomers (PCOs) | 210 | 1.4 | 40 |
Table 4 — Comparison of the effect on collagen fibers of the bioflavonoids with that of proanthocyanidins (OPC)
The results show that the bioflavonoids have no effect as far as crosslinking and stabilizing collagen are concerned. Although catechin does indeed crosslink and stabilize collagen, the PCOs (OPC) are over 4.5 times more effective than catechin. Furthermore, even though the collagen fixes 50% more of the condensed tannins than PCOs (OPC), the PCOs are three times as effective at stabilizing the collagen fibers. The conclusion is that the molecular configuration of the PCOs (OPC) is optimal for the stabilization of collagen.
4.4 Free Radical Trapping Effect
The in vitro free-radical trapping effect of the OPC can be checked in several ways. The tetrazolium nitroblue (TNB) test12 is particularly effective at evaluating the effect of inhibition of superoxide radicals O2-. In this test oxygen radicals have the property of reducing TNB into formazan blue, the quantity of which can be colorimetrically determined at 560 nm. In the presence of antioxidants operating as O2– scavengers, reduction of the TNB is inhibited, which is demonstrated quantatively by a reduction of absorption at 560 nm. Table 5 shows the amount of inhibition obtained with a variety of substances.
| SUBSTANCE | % INHIBITION |
|---|---|
| Catechin-Catechin Dimer | 78 |
| Epicatechin-Catechin Dimer | 72 |
| Epicatechin-Epicatechin Dimer | 63 |
| Malvoside | 32 |
| Epicatechin | 30 |
| Catechin | 25 |
| Chlorogenic Acid | 25 |
| Rutoside | 24 |
| Cafeic Acid | 20 |
| Ascorbic Acid | 4 |
Table 5 — Comparison of In Vitro Inhibition of Oxygen Radicals by OPCs With Various Other Substances
The results show that the dimeric proanthocyandins are nearly 20 times more effective than vitamin C at trapping oxygen radicals, and are greater than two times more effective than the bioflavonoids and the monomers.
Another similar study was reported by Masquelier11A, in which the NBT test was used to compare the ability of various substances to inhibit superoxide radicals, and the results are shown in Table 5A below. One sees that the the oligomeric proanthocyanidins are about 20 times more effective at trapping oxygen radicals than some of the commonly used bioflavonoids.
| NATURE OF THE SCAVENGER INCLUDED IN THE COMPARISON | CONCENTRATION PRODUCING 50% INHIBITION (g/l) |
|---|---|
| Procyanidolic oligomers (from grape seeds) | 0.046 |
| Conventional citroflavonoids | 0.900 |
| Hesperidine methyl chalcone | 1.000 |
Table 5A — Comparison of In Vitro Inhibition of Oxygen Radicals by Procyanidolic Oligomers with other flavonoids
Another more recent and complete test compared the free-radical scavenger activity of procyanidolic oligomers and anthocyanosides with respect to superoxide anion and lipid peroxidation11B. In this study the NBT test was used for comparing superoxide activity. Lipid peroxididation induced by ascorbate and the Fe2+ – ADP complex was assessed in boiled rat liver microsomes. This system produces the hydroxyl radical OH-, which reacts with polyunsaturated fatty acids in microsomal membranes causing the uprooting of a H atom and the subsequent triggering of a cycle of fatty acid self-oxidation. The test is based upon the assay of malondialdehyde (MDA) released as a result of the degradation of lipoperoxides. The results of this study are summarized in Table 5B. This study shows the clear superiority of grape seed procyanidolic oligomers (OPC) as free-radical trappers.
| SUBSTANCE | LIPID PEROXIDATION INHIBITION, mg/ml |
SUPEROXIDE INHIBITION IC50, mg/ml |
COMMENTS |
|---|---|---|---|
| Vitis Vinifera L. (PCO) (grape seeds) | 0.016 | 0.010 | Grape Seed Extract |
| Cupresses Sempervirens L. (PCO) | 0.075 | 0.041 | Extracted from berries |
| Vitis Vinifera L. (AC) (juice) | 0.090 | 0.039 | |
| Vaccinium Myrtillus (AC) (bilberry) | 0.095 | 0.041 | |
| Ribes Nigrum (AC) | 0.16 | 0.047 | black currant extract |
| Cyanidanol (reference monomer) | 0.004/0.018* | 0.051/0.019* | *ref 14 |
| Ginkgo Biloba | 0.25 | ref 13 | |
| BHA | 0.084* | 0.054* | *ref 14 |
| Chlorpromazine | 0.015* | 0.031* | *ref 14 |
| d-Alpha Tocopherol | 0.034* | *ref 14 |
Table 5B — Comparison of Antioxidant Properties of Various Oligomeric Proanthocyanidins, Anthocyanidins, and Other Substances With Respect to the Inihibition of Superoxide Radicals and Lipid Peroxidation. IC50 Values are the Concentrations in mg/ml Needed to Inhibit 50% of the Free-Radical Reactions.
Source: M.T. Meunier, E. Duroux, P. Bastide, Plantes medicinales et phytotherapie, 1989, Tome XXIII, n.4, p.267-274.
Notes: PCO = oligomeric proanthocyanidins AC = anthocyanidins
* references are from the source article
Furthermore, using human umbilical cord and chicken embryo vascular tissue cultures the free-radical scavenging effect of pine bark extract was checked12A. Under certain conditions, these tissue cultures degenerate rapidly and in particular show destruction of the membrane phospholipids caused by oxygen radicals. When pine bark extract was added to the medium, the tissues cultures were maintained in a normal histologic condition.
These results indicate that PCOs/OPCs have very potent free-radical scavenging effects.
4.5 In Vivo Tests of the Effect of Pine Bark Extract on Capillary Resistance
Most of the studies described above were done in vitro or on animals. We will now present the results of a clinical study carried out with a total of 45 patients including 8 controls.13 The patients were suffering from skin diseases or phlebological illnesses (eczema, ulcerated varicose veins, etc.). Various bioflavonoids and pine bark extract were used to conduct this study. Capillary resistance was measured by means of a Parrot angiosterrometer which allows one to ascertain with precision the amount of suction which causes purpura (red spots from broken capillaries) to appear on the skin.
In the first part of the study, patients were given a single 100 mg dose of pine bark extract and the capillary resistance was periodically measured over a 120 hour time inteval. The results are provided in Table 6.
Hours immediately following administration (top) in relation to mean value of capillary resistance (bottom)
| Hours Immediately Following Administration | |||||||||
| 0 | 1 | 3 | 6 | 10 | 24 | 48 | 72 | 96 | 120 |
| Mean Value of Capillary Resistance | |||||||||
| 15 | 26 | 33 | 40 | 21 | 30 | 37 | 44 | 42 | 41 |
Table 6 — Measurements of capillary resistance over a 120 hour time interval in 26 patients after the administration of a single dose of 100 mg pine bark extract
It is observed that the mean capillary resistance increases rapidly peaking initially after 6 hours, then decreases and finally increases to its maximum value and remains at this high level. The term “diphasic” has been applied to this increasing, then decreasing, and finally increasing effect on the capillary resistance.
The next part of the study looked at the effect on the capillary resistance of several other well-known bioflavonoids over a 120 hour period and compared the results with those obtained with pine bark extract. The results are displayed in Table 7.
| SUBSTANCE | NUMBER OF PATIENTS | NUMBER OF MONOPHASIC CURVES |
NUMBER OF DIPHASIC CURVES |
|---|---|---|---|
| Pine Bark Extract | 26 | 2 | 24 |
| Citroflavonoids | 4 | 4 | 0 |
| Hesperidin | 4 | 4 | 0 |
| Trihydroxyethylrutin | 3 | 3 | 0 |
Table 7 — Comparison of the Number of Diphasic and Curves for Various Bioflavonoids and Pine Bark Extract
It is clear from the results shown in Table 7 that the traditional bioflavonoids do increase the capillary resistance for a short period of time, but none of those tested show the secondary long-term increase of pine bark OPC. Obviously there is an effect from the pine bark OPC that is not inherent in the other traditional bioflavonoids.
Table 8 shows the average percentage increase in capillary resistance over the first 72 hours after administration of the tested substance. This result indicates the almost threefold factor of effectiveness for pine bark OPCs as compared to the other bioflavonoids.
| SUBSTANCE | NUMBER OF PATIENTS | AVERAGE INCREASE OF CAPILLARY RESISTANCE DURING 72 HOURS (% of starting value) |
|---|---|---|
| Flavonoids | 11 | 56 |
| Pine Bark OPC | 26 | 140 |
| Placebo | 8 | 3 |
Table 8 — Mean Value of Increase of Capillary Resistance After 72 Hours for a Single Dose of the Test Substance Type
Masquelier has concluded that the initial increase in capillary resistance shown with all of the bioflavonoids and oligomeric proanthocyanidins (OPC) is due to the effect that all of these substances have on inhibiting the enzyme Catecholamine O-Methyl Transferase (COMT) which is responsible for breaking down adrenaline in the body. The prolonged life of adrenaline caused by the flavonoids allows it to decrease capillary permeability (equivalent to increasing capillary resistance). The longer-term effect of increased capillary resistance shown only by OPC in the experiments comes about from the effect that the oligomeric proanthocyandins have upon Vitamin C. The PCOs/OPCs, which are powerful reducing agents, work with glutathione to reduce dehydroascorbic acid back to ascorbic acid within the tissues, which in turn is responsible for the increased capillary resistance by allowing the body to build or rebuild the collagen in the basement membranes of the capillaries.
4.6 Effects of Oligomeric Proanthocyanidins on Histamine Formation
During the initial stages of inflammation, damaged tissues release several chemical substances that activate the inflammation process. These include enzymes that breakdown or decarboxylate histidine into histamine which then increases the permeability of blood vessels. Middleton has reported that a number of the bioflavonoids inhibit histamine release thereby blocking the process of inflammation14. A study was conducted by Dr. David White at the University of Nottingham in England investigating the effect that pine bark OPC has on histidine decarboxylase14B.
In the study, rat gastric mucosa were assayed in vitro for histidine decarboxylase (HDC) activity. HDC was measured by following the release of 14CO2 from 14C-histamine using the method of Beaven et. al.15. The results showed that HDC activity was inhibited in a dose dependent manner by OPC. An in vivo study was conducted by measururing HDC activity in the gastric mucosa of rats which had been given pine bark extract in their diet for 5 weeks at levels of 5 and 50 mg per kilogram of bodyweight. The HDC activity in animals treated at both high and low doses were reduced compared with controls, and, although this was not a very sensitive test, it confirms the in vitro results.
4.7 Effect of OPCs and Catechins on Collagenases and Elastases
Both in vivo and in vitro studies have provided evidence that the binding of oligomeric proanthocyanidins (OPC) to elastin affects its rate of degradation by elastases16. In these studies OPCs and (+) catechin bound to insoluble elastin markedly affected its rate of degradation by elastases. Insoluble elastin pretreated with PCO was resistant to the hydrolysis induced by both porcine pancreatic and human leukocyte elastases. (+) Catechin-insoluble elastin complexes were partially resistant to the degradation induced by human leukocyte elastase but were hydrolyzed at the same rate as untreated samples by a constant amount of pancreatic elastase.
These studies emphasize the potential effect of these compounds in preventing degradation by elastases as occur in inflammatory processes.
In another study17 treatment of radioactively labeled guinea-pig skin collagen or calf collagen with the bioflavonoid (+) – catechin makes the collagen resistant to the action of mammalian collagenase but not to the action of bacterial collagenase…..Since incubation of the mammalian enzyme with (+) – catechin does not inhibit its activity, it is postulated that (+) – catechin binds tightly to collagen and modifies its structure sufficiently to make it resistant to enzyme degradation.
4.8 Effect of OPC on Blood Vessels
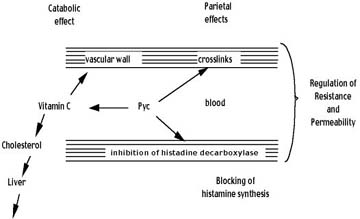
Figure 4 summarizes the ways that OPC protect the blood vessels, especially the capillaries. Regulation of vessel resistance and permeability occur in three separate ways:
Figure 4 — Summary of How OPC help protect the Blood Vessels
-
The OPC crosslink the collagen fibers in the vessel basement membranes which make them stronger and less permeable.
-
The OPC inhibit the production of histamine which prevents the histamine associated increase in permeability. In addition, OPC allow adrenalin to function for an extended time period by blocking the COMT enzyme, thereby allowing for increased vessel resistance.
-
By protecting and carrying vitamin C to the basement membranes, vitamin C is enabled to function more effectively in the production of the collagen needed to keep the vessels strong.
Because much of the cholesterol produced in the body is broken down by Vitamin C, the protective effect that OPC have on Vitamin C indirectly helps the body to reduce high levels of cholesterol which may adversely affect the vessels by plague buildup on the walls.
Some of the effects described above are properties of the common bioflavonoids, however, OPC have more potent as well as additional effects when compared to the common bioflavonoids. Based on the research, it can be expected that on an overall basis, OPC will be many times more effective at protecting the blood vessels than the common bioflavonoids.
REFERENCES
1. Walker, Morton, “Antioxidant Nutrients – Properties of the Most Powerful Antioxidant Nutrient Known to Man: Pycnogenol™.” The American Raum & Zeit, Volume 2, Number 3, 1991, 24–27.
2. Walker, Morton, “Antioxidant Properties of Pycnogenol.” Townsend Letter for Doctors, August/September 1991, 616–19.
3. Masquelier, J., Michaud, J., Laparra, J., Dumon, M.C., “Flavonoides et pycnogenols.” International Journal for Vitamin and Nutrition Research, 49, No3, 307–11, 1979.
4. Masquelier, Jack, United States Patent Number 3,436,407, April 1969.
5. Bate-Smith, E.C., and Swain, T., “Identification of Leuco-anthocyanidins as ‘Tannins’ in Food.” Chemistry and Industry, 1953, 377–78.
6. Masquelier, Jack, “Pycnogenols: Recent Advances in the Therapeutical Activity of Procyanidins. Natural Products as Medicinal Agents.” Beal, J.L. and Reinhard, E., Eds., Supplement of Plant Medica, Journal of Medicinal Plant Research and Journal of Natural Products, LLoydia, July 1980, 243–55.
7. Carper, Jean, The Food Pharmacy. Bantam Books, 1988, 84–90.
8. Thompson, R.S., Haslam, J.E., and Tanner, R.J., Plant Proanthocyanidins. Part I. Introduction; the Isolation, Structure, and Distribution in Nature of Plant Procyanidins, J.C.S. Perkin I, 1972, 1387–90.
9. Weinges, K. and Freudenberg, “Condensed Proanthocyanidins from Cranberries and Cola Nuts.” Chemical Communications, No. 11, 1965, 220–21.
10. Weinges, K. et al., Procyanidine aus Fruchten, Zur Kenntnis der Proanthocyanidine, X, Liebigs Ann, Chem., Bd. 711, 1968, 193.
11. Masquelier, Jack, “Pycnogenols: Recent Advances in the Therapeutical Activity of Procyanidins, Natural Products as Medicinal Agents,” Beal, J. L. and Reinhard, E., Eds., Supplement of Plant Medica, Journal of Medicinal Plant Research and Journal of Natural Products, LLoydia, July 1980, 243–55.
11A. Masquelier, J, “Procyanidolic Oligomers (leucocyanidins).” Parfums, cosmetiques, aromes, n.95, octobre–novembre, 1990.
11B. Meunier, M.T., Duroux, E. and Bastide, P., “Free-Radical Scavenger Activity of Procyanidolic Oligomers and Anthcyanosides with Respect to Superoxide Anion and Lipid Peroxidation.” Plantes medicinales et phytotherapie, Tome XXIII, No4, 1989, 267–74.
12. Nishikimi, M., Rav, N.A., and Yagi, K., Biochem. Biophys. Res. Commun., 46, 1972, 849–54.
12A. Masquelier, Jack, Plant Extract With a Proanthocyanidins Content as Therapeutic Agent Having Radical Scavenger Effect and Use Thereof, United States Patent Number 4,698,360, October 6, 1987.
13. Masquelier, J., Pharmacodynamics: Human Pharmacology (Part IV A) No. 24.
14. Middleton, Elliot Jr., The Flavonoids. TIPS, August 1984, 335–38.
14B. David White, PhD, private communication, October 1990.
15. Beaven, et. al., Anal. Biochem. 84, 638–41.
16. Tixier, Godeau, Robert, and Hornbeck, “Evidence by in vivo and in vitro Studies that Binding of Pycnogenols to Elastin Affects its Rate of Degradation by Elastases.” Biochemical Pharmacology: Vol. 33, No. 24, 3933-39, 1984.
17. Kuttan, R., Donnelly, P., and DoFerrante, N. “Collagen Treated with (+) – Catechin Becomes Resistant to the Action of Mammaliam Collagenase.” Experienta 37 (1981), Birkhauser Verlag, Basel (Schweiz).
Antioxidant Formula, Diabetes Support Formula, Eye & Vision Formula, Joint Health Formula, Chewable Kid’s Mighty-Multi, Hank & Brian’s Mighty Multi-vite!, Mini Multi, Multi Two, OPC-C, PRO-C, Prolyt, Rejuvenate! Pro, Rejuvenate! Berries & Herbs, Rejuvenate! Strawberry-Peach Protein Plus.


1 comment